Search results
Search for "binding constant" in Full Text gives 81 result(s) in Beilstein Journal of Organic Chemistry.
Switchable molecular tweezers: design and applications
Beilstein J. Org. Chem. 2024, 20, 504–539, doi:10.3762/bjoc.20.45

- form is able to bind diamine guests between the two porphyrins in the apical position for each zinc center. However, it has been observed that the closing can also be induced directly by diamine guests such as DABCO. This system exhibits an allosteric response with positive cooperativity as the binding
- constant is higher for the second complexation than the first. The authors later reported a similar system with triazole linkages between the porphyrin and the indoles that act as binding sites for the metal (square planar complex) [76]. These tweezers present a fluorescence quenching upon copper(I
Photoinduced in situ generation of DNA-targeting ligands: DNA-binding and DNA-photodamaging properties of benzo[c]quinolizinium ions
Beilstein J. Org. Chem. 2024, 20, 101–117, doi:10.3762/bjoc.20.11
- binding constant reported for the 8,9-dimethoxybenzo[c]quinolizinium (Kb = 1.2 × 105 M−1) [20]. The slightly lower binding constants of derivatives 3c, 3e, and 3g as compared with the one of compound 3f, may be explained by the larger substituents of the former ligands, which cause more steric repulsion
Tying a knot between crown ethers and porphyrins
Beilstein J. Org. Chem. 2023, 19, 1630–1650, doi:10.3762/bjoc.19.120

- anion. The formed receptor: ion-pair 1:1 complex 4-CsF was stable in solution, as evidenced by 1H NMR spectroscopy. The binding constant Ka = 3.8·105 M−1 in CHCl3/MeOH 9:1 was reported. The XRD analysis in the solid state provided further proof of the binding mode, demonstrating the significant
- separation between the cation and anion. Sessler and co-workers introduced a similar ion-pair receptor, in which the calix[4]arene-strapped calix[4]pyrrole 5 demonstrated an additional binding mode of CsF (Figure 5). The binding constant Ka = 1.3·104 M−1 in CHCl3/MeOH 9:1 was reported [101][102]. This
CO2 complexation with cyclodextrins
Beilstein J. Org. Chem. 2023, 19, 1021–1027, doi:10.3762/bjoc.19.78
- yields was sufficient time for the crystallization, while the pressure was found less important. From the binding constant measured below and the 1:1 stoichiometry (Kg = 0.18 bar−1, Table 2) we know that 1/2 to 3/4 of the cyclodextrin is filled with CO2 at the pressures used as shown in the 5th column of
- characteristic water absorption at 3200–3600 cm−1 and the C=O band of CO2 at 2350 cm−1 during the both weigth losses but mainly CO2 at the first lump and predominantly water at the second loss. This also suggest a comparatively weak binding of the CO2. We determined the binding constant for CO2 to cyclodextrins
- reference spectrum without cyclodextrin, we determined the gas binding constants from non-linear regression of bound CO2 versus CO2 pressure as shown for 1 in Figure 5. This gave the gas binding constant Kg = [CD·CO2]/[CD]PCO2 given in Table 2 in bar−1. This constant gives the fraction of CD bound CO2 in
Preparation of β-cyclodextrin-based dimers with selectively methylated rims and their use for solubilization of tetracene
Beilstein J. Org. Chem. 2022, 18, 1596–1606, doi:10.3762/bjoc.18.170
- substituted rims are partially methylated CDs. Methylation reduces the formation of intramolecular hydrogen bonds, enhancing CDs water solubility, and also extends the hydrophobic cavity, thus improving its binding potential. A substantial increase of binding constant (K) for per-6-methylated CD compared to
Microelectrode arrays, electrosynthesis, and the optimization of signaling on an inert, stable surface
Beilstein J. Org. Chem. 2022, 18, 1488–1498, doi:10.3762/bjoc.18.156

- chosen as the ligand for Gαi1 because it was known to bind the target with a binding constant of KD = 60 nM [21]. The binding data shown in Figure 5 compared interactions between Gαi1 and two surface bound peptides; R6A (MSQTKRLDDQLYWWEYLC) and a scrambled R6A sequence (QLSEDTYLLMRWDYWQK). The two
- never leaves the surface. The surface does not recover its original conductivity and the experiment shows a binding constant that is greater than either of the individual interactions. Alternatively, the surface-bound substrate could lead to avidity events where more than one of the ligands binds to the
- curve. Once again, the binding curve measured in this manner was amplified relative to the known nanomolar binding constant for the interaction. When three times the reaction time was used for the placement reaction (12 cycles), the binding curve was amplified out of the window. In this case, no signal
Synthesis of a new water-soluble hexacarboxylated tribenzotriquinacene derivative and its competitive host–guest interaction for drug delivery
Beilstein J. Org. Chem. 2022, 18, 539–548, doi:10.3762/bjoc.18.56
- . Subsequently, the binding stoichiometric ratio of the complex was determined to be 1:1 by the Job plot method (Figure 2b and Figure S11 in Supporting Information File 1) through fluorescence spectrometry, and the host–guest binding constant between TBTQ-CB6 and DOX was calculated to be Ka = (6.81 ± 0.33) × 104
- fluorescence titration experiments and the corresponding nonlinear fitting curve (Figure 4c and 4d), the binding constant Ka for TBTQ-CB6 and SM was determined to be (5.09 ± 0.98) × 105 M−1. Compared with the binding constants determined for TBTQ-CB6MV and TBTQ-CB6DOX, the higher binding constant of TBTQ-CB6SM
Host–guest interaction and properties of cucurbit[8]uril with chloramphenicol
Beilstein J. Org. Chem. 2021, 17, 2832–2839, doi:10.3762/bjoc.17.194
- between the assemblies. Figure 4 and Table 1 show the exothermic isotherms and thermodynamic constants obtained for the titration of CPE with Q[8] interaction using ITC. From the data, it can be seen that the reaction was enthalpy driven and its binding constant was 8.057 × 105 L/mol. 1H NMR spectroscopy
19F NMR as a tool in chemical biology
Beilstein J. Org. Chem. 2021, 17, 293–318, doi:10.3762/bjoc.17.28

- dissociation binding constant of ligands (Kd) determined from the changes in the chemical shift of the labelled nuclei within the protein. Intuitively, the largest chemical shift perturbations are expected to be seen for residues in the closest proximity to the bound ligand. An additional advantage of this
The fluorescence of a mercury probe based on osthol
Beilstein J. Org. Chem. 2021, 17, 22–27, doi:10.3762/bjoc.17.3
- fluorescence, UV–vis spectrophotometry, mass spectrometry, and 1H NMR spectroscopy, and the recognition mechanism is discussed. The results showed that OST and Hg2+ can form a complex with a stoichiometric ratio of 1:1. The binding constant was 1.552 × 105 L∙mol−1, having a highly efficient and specific
Molecular basis for protein–protein interactions
Beilstein J. Org. Chem. 2021, 17, 1–10, doi:10.3762/bjoc.17.1

- thermodynamic properties, including the binding constant Kb, the reaction stoichiometry (n), the observed molar calorimetric enthalpy ΔHobs, the entropy ΔS, the heat capacity of the binding ΔCp,obs, and the change in the Gibbs free energy ΔG. As a result, ITC is used to provide a complete thermodynamic
Selected peptide-based fluorescent probes for biological applications
Beilstein J. Org. Chem. 2020, 16, 2971–2982, doi:10.3762/bjoc.16.247

- 540 nm in presence of 10 µM ATP (Figure 2B). The ATP binding constant is calculated to be 2.2 × 105 M−1. A fluorescence job plot between 1 and ATP confirms an 1:1 binding stoichiometry between ATP and probe 1. The fluorescence response follows the following order ATP > GTP > UTP > CTP > TTP > PPi
- of the PDA liposomes to about 48% of the initial value (Figure 10B). The calculated binding constant from Stern–Volmer analysis of fluorescence titration is 1.5 × 106 M−1. This system is very selective towards LPS as other analytes, including nucleotides, anionic sugars, and ctDNA did not increase
Synthesis and investigation of quadruplex-DNA-binding, 9-O-substituted berberine derivatives
Beilstein J. Org. Chem. 2020, 16, 2795–2806, doi:10.3762/bjoc.16.230
- between the length of the alkyl chain n and the binding constant Kb cannot be deduced from these data, as has been done with other alkyl-substituted berberine derivatives [38][39][40][41][42]. In the latter cases, however, the alkyl chains were substituted with positively charged functionalities that
- other parameters such as binding-site size, cooperativity between ligands, ionic strength, the enthalpy of the DNA denaturation, and on the binding constant and enthalpy of the ligand binding at the melting temperature. However, the binding constant is determined at temperatures below Tm and the
- different quadruplex forms of this particular DNA [50][51]. In the case of the G4-DNA 22AG, the stabilization by the ligands 4a–e is more consistent with the binding constants, as both sets of data indicate a moderately high binding constant and a good stabilization towards thermally induced unfolding
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- binding properties, isothermal titration calorimetry is an advantageous method as it yields the binding stoichiometry, the binding constant Ka and the binding enthalpy ΔH0 in one measurement. From these data, the Gibbs free binding energy ΔG0 and the binding entropy ΔS0 can be calculated. Pseudo[2
- ion, which resulted in binding constants in the optimal range for ITC titrations [52][53]. The even more weakly coordinating tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (BArF24−) anion exemplarily served for comparison to study the influence of the anion on the binding constant. Two different
- binding constant, but enthalpic and entropic contributions have not yet been studied [50][51][55]. As PF6− is more strongly coordinating than BArF24−, a larger fraction of A1·PF6 ion pairs is present in nonpolar solvents such as DCE. Upon complexation, the ion pair of A1·PF6 must dissociate, releasing PF6
NMR Spectroscopy of supramolecular chemistry on protein surfaces
Beilstein J. Org. Chem. 2020, 16, 2505–2522, doi:10.3762/bjoc.16.203

- simultaneously and thus induce aggregation [23][24][25][27][82]. This behavior does not interfere with identifying the ligand binding site, but it makes it difficult to determine a binding constant from the chemical shift perturbations since fitting a binding curve requires sufficient saturation of the signal
Host–guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A
Beilstein J. Org. Chem. 2020, 16, 2332–2337, doi:10.3762/bjoc.16.194

- –guest interactions between oroxin A (OA) and cucurbit[8]uril (Q[8]) using 1H NMR, MS, UV–vis and IR spectroscopy. The results showed that OA and Q[8] formed an inclusion compound (OA@Q[8]) with a molar ratio of 1:1 and a binding constant of 1.299 × 107 L·mol−1. In addition, the effect of Q[8] on the
- transition of the absorbance of the system. Upon further addition of Q[8], the absorption value of the system tended to be constant, indicating the formation of a 1:1 complex with a binding constant K = 1.299 × 107 L·mol−1. The result of the Job’s plot also confirmed the combination of Q[8] and OA in a 1:1
- ] formed a host–guest complex in a ratio of 1:1. The aglycone of OA entered the cavity of Q[8] and the glucose was located at the portal of Q[8], with a binding constant of 1.299 × 107 L·mol−1. The solubility of oroxin A was increased 22.47-fold when the concentration of the added Q[8] was 1.0 × 10−4 mol·L
Naphthalene diimide bis-guanidinio-carbonyl-pyrrole as a pH-switchable threading DNA intercalator
Beilstein J. Org. Chem. 2020, 16, 2201–2211, doi:10.3762/bjoc.16.185

- 2). The detailed analysis of results (Table 2) revealed that at neutral conditions (pH 7) compound 4 showed at least one order of magnitude lower binding constant for ds-DNA and ds-RNA in comparison to the affinity at pH 5, agreeing well with the thermal denaturation results (Table 1), which can be
- ], agreeing well with threading intercalation. The 4/GC-DNA complex also showed a strong ICD band at 270–290 nm, typical for NDI intercalation [26][27], and the binding constant was exceptionally high. The absence of an ICD band at 350–420 nm suggested a different angle of the NDI longer axis in respect to
- calorimetry experiments (ITC), which allowed us to determine all thermodynamic components simultaneously in a single experiment (the equilibrium binding constant (Ka), reaction Gibbs free energy of binding (ΔrG), reaction enthalpy (ΔrH), reaction entropy (ΔrS), and the stoichiometry (n) of the complex formed
Reversible photoswitching of the DNA-binding properties of styrylquinolizinium derivatives through photochromic [2 + 2] cycloaddition and cycloreversion
Beilstein J. Org. Chem. 2020, 16, 111–124, doi:10.3762/bjoc.16.13
- isotherms, and fitting of the experimental data to an established theoretical model [73] gave the corresponding binding constants Kb (cf. Supporting Information File 1). Thus, the largest binding constant was determined for the dimethylamino-substituted styrylquinolizinium derivative 3a (Kb = 2.6 ± 0.1
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
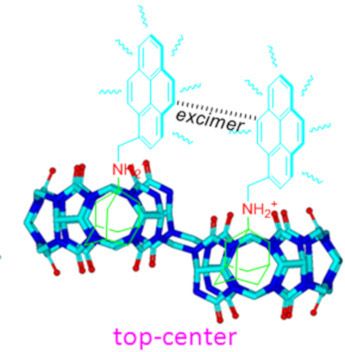
- effect of the carbonyl-rimmed portal). Isothermal titration calorimetry results reveal a 2:1 G1/host-1 stoichiometry with a binding constant (Ka) of 1.32 × 105 M−1 (Table 1). These observations clearly indicate that host-1 recognizes and prefers to include the ADA moieties in both of the identical
Reversible end-to-end assembly of selectively functionalized gold nanorods by light-responsive arylazopyrazole–cyclodextrin interaction
Beilstein J. Org. Chem. 2019, 15, 1407–1415, doi:10.3762/bjoc.15.140

- 20-fold higher binding constant to β-CD (6.5 × 104 M−1) [32] than unmodified azobenzenes (2.5 × 103 M−1) [33]. Therefore, the competitive binding of CTAB to β-CD has been applied previously, e.g., in sensing applications [34] or to enhance the water solubility of a cyclodextrin polymer [35]. On
- competitive interaction with β-CD since it has a higher binding constant than the AAP moieties. The success of the consecutive ligand exchange strategy was verified by ζ-potential measurements. The selective end-to-end assembly of AuNR was evidenced by UV–vis and TEM measurements. The LSPR shift could
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- 103 M−1 to Fl, RB and TPPS, accompanied by a slight fluorescence enhancement [10][33][34]. One extraordinary example is the complexation between permethylated β-CD (PMCD) and TPPS. A specific 2:1 PMCD–TPPS complex was formed with an exceptionally high binding constant which was even too large to be
Efficient resolution of racemic crown-shaped cyclotriveratrylene derivatives and isolation and characterization of the intermediate saddle isomer
Beilstein J. Org. Chem. 2019, 15, 1339–1346, doi:10.3762/bjoc.15.133

- highest binding constant for Xe encapsulation in organic solvents known today [25]. However, unless fixed like in a cryptophane CTVs are usually flexible enough to undergo ring inversion which is synonymous to a racemization in case of chiral CTVs. This intramolecular process presumably proceeds via less
Fabrication, characterization and adsorption properties of cucurbit[7]uril-functionalized polycaprolactone electrospun nanofibrous membranes
Beilstein J. Org. Chem. 2019, 15, 992–999, doi:10.3762/bjoc.15.97

- blue (MB) is frequently used as a routine model dye molecule to test the adsorption capability of materials. More importantly, the binding constant of MB with CB[7] is as high as 107 M−1 in aqueous solution [33]. However, due to the considerable solubility of CB[7] in water, the adsorption experiment
Silanediol versus chlorosilanol: hydrolyses and hydrogen-bonding catalyses with fenchole-based silanes
Beilstein J. Org. Chem. 2019, 15, 167–186, doi:10.3762/bjoc.15.17

- ability of binding ions or molecules via hydrogen bonds. To investigate this ability, UV–vis titration experiments with tetrabutylammonium chloride (TBA-Cl) are carried out, with silanediol 1 as a reference (Table 6) [47][75]. For BIFOXSi(OH)2 (9, Figure 14, Figure 15) a binding constant of 5274.9 M−1 (13
- %) for chloride is determined, which is in the same order as di(1-naphthyl)silanediol (1, Figure 1) with 4688.0 M−1 (5%, Table 6). The binding constant of chlorosilanol 8 (Figure 13) for chloride is 451.1 M−1 (4%). Thus chlorosilanol 8 and silanediol 9 are feasible for anion binding of chloride. Counter
- better than BIFOXSiCl(OH) (8) which is in accordance with the determined binding constant for chloride (Table 6, Table 9). With BIFOXSiCl(OH) (8) a yield up to 60% is isolated (Table 9, entry 3), but as racemate. BIFOXSi(OH)2 (9) catalyses the reaction with good yields up to 73% and an ee value of 12
Calixazulenes: azulene-based calixarene analogues – an overview and recent supramolecular complexation studies
Beilstein J. Org. Chem. 2018, 14, 2488–2494, doi:10.3762/bjoc.14.225

- absence of heteroatoms, most commonly hydroxy groups on the “lower” or narrow rim, also limits their “pre-organizational” potential for supramolecular binding, this being of particular interest to us. We now report that we have succeeded in extracting binding constant data from a solution-state UV–vis
- are shown in Table 1. No easily discernable significant correlation between the interaction energies and the experimentally measured binding constants can be discerned for the three halide salt complexes; the highest IE (−337.805 kJ mol−1) was found for the chloride which also had the highest binding
- constant but the corresponding values for the bromide and iodide salts showed no such correlation. The correlations between the IEs and binding constants for the tetrafluoroborate salts, however, are more easily discernable and have the same trends in the order of TBABF4 > TEABF4 > TMABF4. The counterion

















































































































































































































































































































